Compliance within the company
The Group Compliance Officer and other specialists from our Group function Compliance are in charge of defining our compliance agenda, which we continuously adjust in response to current requirements. Local and regional compliance officers are responsible for implementing compliance measures within their respective subsidiaries. In 2015, we appointed a compliance officer for each of our business sectors (Healthcare, Performance Materials and Life Science) in an effort to better address the needs of each specific business sector. In the course of regular compliance audits, our Group function Internal Auditing reviews the implementation of compliance procedures within our facilities.
We regularly hold workshops to inform our employees about new compliance requirements. In 2015, a total of 20,404 persons participated in anti-corruption courses. In 2014, we designed an e-course to introduce our new anti-corruption guideline, and in 2015 we had the course translated into 16 different languages. This has allowed numerous employees to complete the course in their local language. In 2015, we also developed a special e-course for our pharmaceutical business that explains the guidelines specific to this field.
Personnel training on anti-corruption guidelines
Number of persons trained
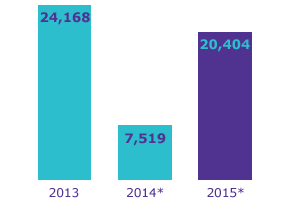
* Includes contractors and supervised workers (e.g. temps) who were trained on our anti-corruption guideline (2,023 in 2014; 3,026 in 2015).
Stringent requirements placed on our business partners
Effective compliance management does not stop at our front door, which is why we set the highest standards for the conduct of our business partners. The selection process for business associates is governed by our Global Business Partner Risk Management Guideline. This policy stipulates that Merck KGaA, Darmstadt, Germany shall only do business with partners who comply with all applicable laws; who do not engage in bribery; who adhere to environmental, health and safety guidelines; and who refuse to tolerate discrimination. Furthermore, we require our partners to demonstrate a commitment to internationally recognized human rights and labor standards, as well as to the compliance standards defined in our Code of Conduct. We also audit existing business relationships, usually when it's time to renew a contract.
If we identify red flags, we may consider rejecting potential business partners or even terminating the business relationship. However, our business associates are frequently willing to modify their structures and processes to meet our stringent compliance requirements. Since implementing this process in 2013, nearly 1,500 business partners have undergone this audit.
In 2015, we introduced compliance training in eight languages for the employees of our business associates. The training is mandatory for anyone who comes into contact with us or our products in the course of their work. It focuses on general compliance, corruption prevention, and competition law.
Transparent business relationships in the pharmaceutical industry
In Europe effective 2016, all non-research related donations made to physicians and healthcare organizations must be disclosed, with the recipients individually named. This is a requirement stipulated by the transparency initiative of the European Federation of Pharmaceutical Industries and Associations (EFPIA). In 2014, our Compliance organization had already initiated the measures necessary to satisfy this disclosure obligation. In 2015, we focused part of our efforts on informing our partners in the health industry just how important this transparency initiative is to us. We also took steps to ensure data quality and data security in all affected countries. Our goal is to disclose the required information by June 30, 2016. A valuable side benefit: Our efforts to promote transparency for our stakeholders have also improved our internal processes.
Fighting corruption hand-in-hand with the Alliance for Integrity
In October 2015, we joined the Alliance for Integrity (AfIn), an initiative founded in October 2015 by the German Agency for International Cooperation (GIZ), the German Global Compact Network, and the Federation of German Industry. The aim of the AfIn is to develop practical procedures to improve the framework for compliance, thereby furthering the fight against corruption. The initiative is concentrating first on local activities in India, Ghana, and Brazil. At the end of November 2015, we took part in a meeting of the AfIn Advisory Group in New Delhi, and in December 2015 we were represented for the first time at a meeting of the organization's steering committee.