We use a structured guideline system to translate our Values and Mission Statements into concrete terms. The purpose is to ensure that all employees know the relevant rules and can apply them in the workplace. Proven management systems ensure that the processes relevant to our CR strategy are steered and monitored systematically.
Group-wide guideline system
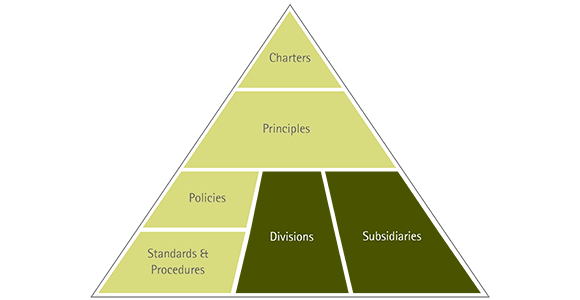
The Group-wide guideline system contains all company guidelines and explains which guidelines apply to which parts of the company. They range from the charters and principles valid for the entire company to specific standards and procedures for divisions and legal entities of the Merck KGaA, Darmstadt, Germany, or for individual sites.
The charters and principles include, for example, our Code of Conduct , our Social Charter and the Access to Health Charter.
Examples of policies in the company are the “Corporate EHS Policy” valid throughout the Group, which establishes the framework for principles, strategies and organizational structures for environment, health and safety at Merck KGaA, Darmstadt, Germany; our Animal Welfare Policy, which describes the treatment of laboratory animals throughout the company; and our guidelines for stem cell and fertility research, which define the ethical framework for our research and development departments.
In addition, we have numerous other division-specific policies, such as the Safety Policy for chemical products, with which we have established global processes for defining, steering and implementing product safety as well as the corresponding management structures.
Our standards specify in concrete terms the provisions from charters, principles and policies for the persons responsible for the operational processes. In the area of company environmental protection, for example, we have standards for protecting water, managing waste, and ensuring warehouse and transport safety. For pharmaceutical marketing, for instance, a standard exists that defines the Group-wide specifications for product advertising and sponsoring.
The rules are kept up-to-date by the relevant departments and are available on the intranet. Managers are responsible for implementation in their respective areas of responsibility. Our employees receive information and training regarding rules that apply to them. In this way, we ensure that they are familiar with both the overarching rules from the charters and principles as well as the concrete specifications that affect their individual range of activities.
Group-wide guideline system
CR management systems
The guideline system that we use to implement corporate responsibility in the Merck KGaA, Darmstadt, Germany is integrated into our management systems. With the help of these systems, we set and steer goals, actions and responsibilities in key action areas. Our management systems are based on standards such as the internationally recognized ISO 9001 and 13458 standards (for quality management, the latter specifically for medical devices), GxP (guidelines for “good working practices in the pharmaceutical industry”) and ISO 14001 (environmental management). The ISO 14001 environmental management system and the ISO 9001 quality management systems are certified at regular intervals by an independent auditing firm. Merck KGaA, Darmstadt, Germany holds group certificates for the quality and environmental management systems.
Other management systems also exist, such as the Group-wide occupational health and safety management system as well as local systems at the sites. Examples of local management systems include wastewater, waste and energy management.
Program for continuous process improvement
Operational Excellence is the Group-wide program Merck KGaA, Darmstadt, Germany introduced in 2006 to continuously improve entrepreneurial processes. The purpose of Operational Excellence is to achieve the most economic and efficient level of operation in all our production facilities. Operational Excellence calls for a working culture that enables continuous improvement and performance enhancement. For this purpose, we leverage the expertise and knowledge of our employees.
Operational Excellence is a continuous process, a program that consists of an annual cycle of four stages: self-assessment and analysis, goal-setting, best practice sharing and implementation of improvement measures.
The program is divided into six management fields: goals, change management, production, logistics, innovation/technology, and employees. These fields in turn contain different bricks, including our Values, employee development, safety culture and EHS, that can be supplemented as needed. Each year, top management defines Group-wide priorities for implementing the bricks. In recent years, the program has focused primarily on the globalization of structures and processes and efficiency improvements. For instance, during the 2011-2012 period, the topic of utility management (with emphasis on energy management) became mandatory to support achievement of our climate protection target. Our individual sites can additionally define their own priorities.

 10
10